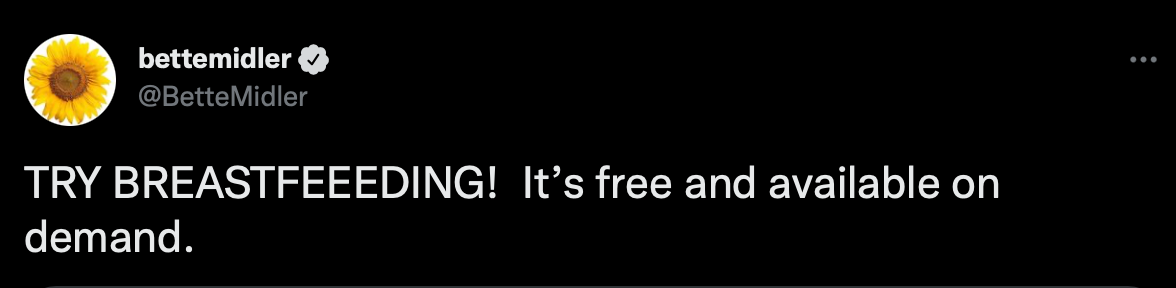
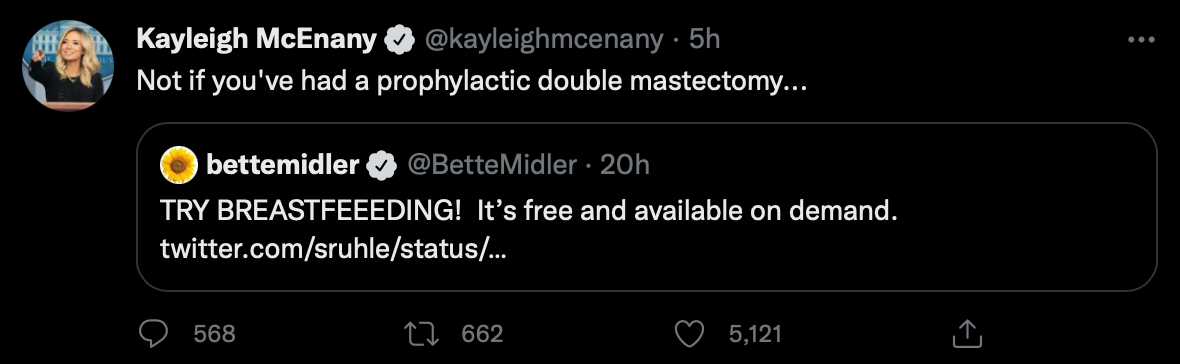
Baby Formula
-
Thanks, George - apparently, there's a rightwing propaganda machine, too.
It makes no sense considering the numbers involved to blame all this on giving it to illegals.
-
@Doctor-Phibes No worries. She hangs around social media for ten minutes, she'll have more solutions than she'll ever be able to use.
@Catseye3 said in Baby Formula:
@Doctor-Phibes No worries. She hangs around social media for ten minutes, she'll have more solutions than she'll ever be able to use.
And that's the truth. The FDA is screaming it's unsafe, but millions of kids have been raised on homemade formula.
Hell, I've got my doctor's notes...IIRC, they were introducing pureed TABLE FOOD at around six weeks...
-
How about "newspaper readers?"
Baby Formula Is Hard to Find. Brands and Stores Are Divided Over Why.
Retailers and formula makers agree that out-of-stocks are a problem. They don’t agree on how severe it is and who is to blame. Chains like Walmart Inc. WMT 0.39%▲ and CVS Health Corp. CVS -0.78%▼ say the manufacturers are having supply issues; formula makers say retailers aren’t getting product to stores once it is delivered.
“The shelves are just bare,” said Derval Kenny, 65, of Rye, N.Y., who has been trying to help find Similac formula for two infant grandsons who live in Connecticut and New Jersey. “To me, there should be an uproar.”
Advertisement - Scroll to Continue
Ms. Kenny, whose grandsons are five and six months old, said she has driven to stores across her county and into neighboring Connecticut and placed an order on Amazon last week that hasn’t yet been delivered. While she hasn’t been able to find the premixed formula bottles that her grandsons use, she has found powdered versions, she said.
The shortages are intermittent and vary based on retailer and location, said Krishnakumar Davey, president of strategic analytics at IRI, a retail research firm. Nationwide, in-stock levels for baby formula and food are slightly higher than for food products overall. However, Mr. Davey said, some of the nation’s 10 largest retailers had more than 20% of baby formula out of stock the week ended Jan. 2, typical of recent months. An out-of-stock rate above 10% is considered a problem, he said.
That was in January.
-
This is one of those things that I had no idea was so stupidly political until I had an infant of my own. What possible reason could anybody have for having an opinion on whether or not other people's kids drink formula?
-
Now, Biden would be more concerned if it was an Ensure crisis...
-
 J Jolly referenced this topic on
J Jolly referenced this topic on
-
Maybe Predient Biden should get involved like Premier Kim does in DPRK
(http://www.vok.rep.kp/index.php/Data_detail_common/getDetail/iee220413006/8/en)
-
Mrs. George and I were on the way to our riding lesson this afternoon, and she asked me to explain the cause(s) of this crisis.
In a tl;dr version, can someone womansplain this to me?
Limited production in the US...? Why?
Limited imports...? Why?
Has this been brewing for a while...? And now you noticed? Should've had a crystal ball, I suppose. -
I think it's the government's business to not get in the way businesses making baby formula.
Besides, I'm the guy that wants to keep babies alive. You're the guy that wants to cut babies to pieces in the womb and then suck them out with a vacuum, to have the bloody body parts splat into a plastic container.
-
I think it's the government's business to not get in the way businesses making baby formula.
Besides, I'm the guy that wants to keep babies alive. You're the guy that wants to cut babies to pieces in the womb and then suck them out with a vacuum, to have the bloody body parts splat into a plastic container.
@Jolly said in Baby Formula:
I think it's the government's business to not get in the way businesses making baby formula.
Consider what happened when a government did not sufficiently got in the way businesses making baby formula:
-
How about "newspaper readers?"
Baby Formula Is Hard to Find. Brands and Stores Are Divided Over Why.
Retailers and formula makers agree that out-of-stocks are a problem. They don’t agree on how severe it is and who is to blame. Chains like Walmart Inc. WMT 0.39%▲ and CVS Health Corp. CVS -0.78%▼ say the manufacturers are having supply issues; formula makers say retailers aren’t getting product to stores once it is delivered.
“The shelves are just bare,” said Derval Kenny, 65, of Rye, N.Y., who has been trying to help find Similac formula for two infant grandsons who live in Connecticut and New Jersey. “To me, there should be an uproar.”
Advertisement - Scroll to Continue
Ms. Kenny, whose grandsons are five and six months old, said she has driven to stores across her county and into neighboring Connecticut and placed an order on Amazon last week that hasn’t yet been delivered. While she hasn’t been able to find the premixed formula bottles that her grandsons use, she has found powdered versions, she said.
The shortages are intermittent and vary based on retailer and location, said Krishnakumar Davey, president of strategic analytics at IRI, a retail research firm. Nationwide, in-stock levels for baby formula and food are slightly higher than for food products overall. However, Mr. Davey said, some of the nation’s 10 largest retailers had more than 20% of baby formula out of stock the week ended Jan. 2, typical of recent months. An out-of-stock rate above 10% is considered a problem, he said.
That was in January.
@George-K said in Baby Formula:
How about "newspaper readers?"
The whistleblower complaint about Abbott labs came forward in February. A month into the Biden presidency.
Abbott and the Food and Drug Administration were alerted to a whistleblower complaint about Abbott's Sturgis infant formula plant as far back as February 2021, ABC News has confirmed.
This complaint, filed with the U.S. Labor Department's Occupational Safety and Health Administration, alleges quality control concerns at Abbott's formula plant in Sturgis, Michigan -- a year before the company's massive recall and shutdown in February 2022 following contamination concerns, which helped exacerbate a nationwide shortage in baby formula, according to sources familiar with the matter.
OSHA received a complaint from a whistleblower on Feb 16, 2021, and sent a copy three days later to the FDA and Abbott, according to a person familiar with the matter.
The complaint raises further questions about when both Abbott and federal health authorities first knew about quality and contamination concerns at the Sturgis plant, and why it took so long for action to be taken.
The OSHA complaint, first reported by WSJ, alleges problems at the Sturgis plant like faulty equipment in need of repair or upgrade and inadequate safety validation for released product.
It was filed several months before similar allegations were made in another whistleblower report, which flagged contamination concerns at the Sturgis plant in October 2021, according to sources familiar with the matter.
-
Now, the rest of the story …
If anyone still cares, Bloomberg published a nice article with timeline of events leading up to and the subsequent resolution of the baby formula shortage of 2022, with hindsight analysis and stories of individuals adversely affected by the tainted formula, etc.
Anyone here still seeing impacts of baby formula shortage on people you personally know?
-
IOW, if the government had done what they were supposed to do, the chances are the company would have been in compliance.
But while local cops, firefighters, grocery clerks and hospital workers did their jobs (some who were fired for not taking the vaccine), the inspectors sat at home and collected their paychecks.
-
IOW, if the government had done what they were supposed to do, the chances are the company would have been in compliance.
But while local cops, firefighters, grocery clerks and hospital workers did their jobs (some who were fired for not taking the vaccine), the inspectors sat at home and collected their paychecks.
@Jolly said in Baby Formula:
IOW, if the government had done what they were supposed to do, the chances are the company would have been in compliance.
… the inspectors sat at home and collected their paychecks.
What policy remedies would you suggest? More stringent inspection standard and more elaborate, higher frequency inspection schedules? Hire more and better quality inspectors? Retrain (and in some cases replace) the existing inspectors? (If you were to replace some inspectors, how would you identify who to replace, and how would you increase the likelihood that the replacements will work better than those replaced?)
Looking into the FDA’s funding history, the usual “the FDA has been chronically underfunded” complaints aside, I also see that the FDA is increasingly reliant on “user fees” (as opposed to Congress appropriated moneys) to fund its operations, meaning they are increasingly reliant on the businesses they inspect/regulate to pay their bills. Does this worry you that the regulator may get too reliant on and too chummy with the regulated? Would you rather see the FDA’s funding to go back to more Congress appropriated funds and rely less on “user fees”?