Why There's No Cure for Alzheimer's
-
A dive into "consensus," funding and review.
https://www.thefp.com/p/where-is-the-cure-for-alzheimers
Neve eventually left the Alzheimer’s field because she was disillusioned by the heavy focus on amyloids. “The amyloid hypothesis is one of the most tragic stories in modern biomedical research,” Neve wrote to me in an email. “The field of Alzhiemer’s disease research has effectively been at a standstill because of that.”
Nobel laureate Dr. Thomas C. Südhof, a professor of molecular and cellular physiology at Stanford Medicine, says medical journals are particularly to blame. Publications in scientific journals, preferably prestigious ones, are make-or-break for scientists’ careers. But the experts who get to decide what is published have a personal incentive not to encourage findings that might run counter to their own beliefs..
All this brings us back to Biogen and Eisai’s new drug, lecanemab—the “gamechanger” amyloid treatment scheduled for possible accelerated approval by the FDA soon. If it comes to market, some analysts predict it could surpass $9 billion a year in sales.
Biogen touted the “highly statistically significant reduction of clinical decline” in a press release at the end of September. So let me explain what a “gamechanger” for Alzheimer’s looks like. For the trial, nearly 1,800 people diagnosed with Alzheimer’s were divided in half, with one group getting lecanemab, the other a placebo.
These patients were then evaluated for their ability to perform activities of daily living, such as feeding and dressing themselves. Their cognitive function, such as the ability to identify common objects and be socially appropriate, was also assessed. In the lecanemab group, the drug slowed cognitive decline among patients by 0.45 points on an 18-point scale. You read that right—less than a one-point improvement in evaluations of patients’ memory, judgment, ability to care for themselves, and other measures. And that’s a slowing of deterioration, not a reversal.
In addition, three patients in the clinical trial are known to have died from bleeding and swelling of the brain that is believed to be a result of the treatment. As an article in Science notes, brain swelling is a well-documented side-effect of medications that attack amyloid.
-
A couple of comments:
- The peer-reviewers/gate keepers affect not only the publication process, but the grant approval process for NIH and organizations, like my own, that use the NIH process (three detailed reviewers score it along several dimensions, present to a larger group who then score based on presentations).
- This seems unavoidable, in that you need people very qualified in the area to see how much sense a grant makes. But the key way to minimize the group-think risk is too make sure you have a diversity of views on those committees. As a (small, not really consequential) example, in our disease community there are believers in inhaled AAT as a potential therapy and non-believers. We have some of each on the grant advisory committee.
- Companies, especially biotechs, tend to be more adventurous in things they'll attempt, at least in the lab. HOWEVER, companies generally don't invest money to understand disease pathways, they let academia figure out how the disease actually works, and they go from there. IOW, they are adventurous in how they ATTACK targets, and even which targets to attack, but they don't try to figure out what the targets are in the first place (in general in my world at least).
- I don't know how common situations are like this, where one pathway gets decades of funding to the exclusion of others, and then turns out to be wrong. Usually it seems to me there are competing camps so one side might be favored, but the others aren't necessarily squelched.
I'd be curious to get Bach's opinion, as oncology is an entirely different beast as far as drug development goes. For example, I work with a guy that used to be in charge of all early stage trials for a big Pharma co, "except oncology".
(Lecanemab got approval today, by the way)
-
‘Disruptive’ science has declined — and no one knows why
The number of science and technology research papers published has skyrocketed over the past few decades — but the ‘disruptiveness’ of those papers has dropped, according to an analysis of how radically papers depart from the previous literature1.
Data from millions of manuscripts show that, compared with the mid-twentieth century, research done in the 2000s was much more likely to incrementally push science forward than to veer off in a new direction and render previous work obsolete. Analysis of patents from 1976 to 2010 showed the same trend.
“The data suggest something is changing,” says Russell Funk, a sociologist at the University of Minnesota in Minneapolis and a co-author of the analysis, which was published on 4 January in Nature. “You don’t have quite the same intensity of breakthrough discoveries you once had.”
Telltale citations
The authors reasoned that if a study was highly disruptive, subsequent research would be less likely to cite the study’s references, and instead cite the study itself. Using the citation data from 45 million manuscripts and 3.9 million patents, the researchers calculated a measure of disruptiveness, called the ‘CD index’, in which values ranged from –1 for the least disruptive work to 1 for the most disruptive.The average CD index declined by more than 90% between 1945 and 2010 for research manuscripts (see ‘Disruptive science dwindles’), and by more than 78% from 1980 to 2010 for patents. Disruptiveness declined in all of the analysed research fields and patent types, even when factoring in potential differences in factors such as citation practices.
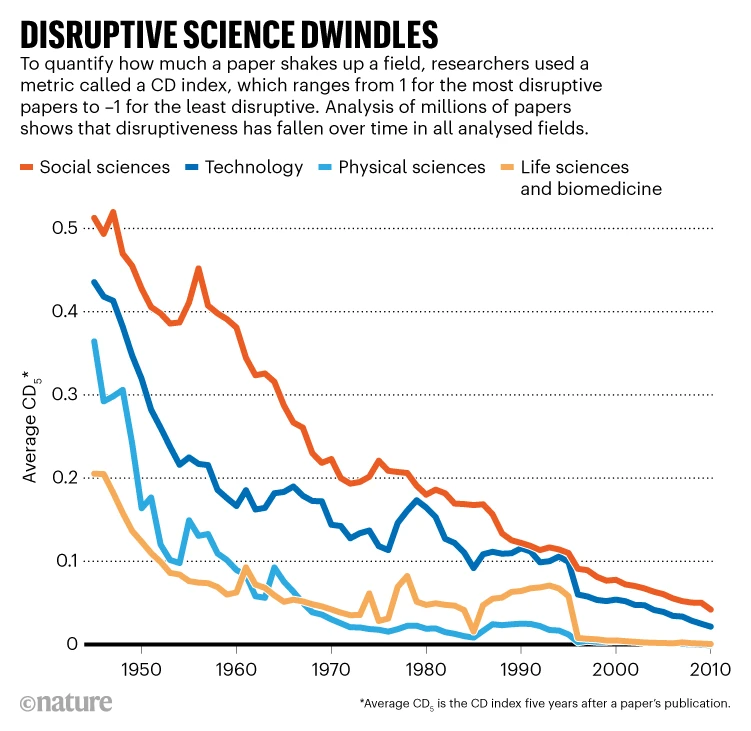
It is important to understand the reasons for the drastic changes, Walsh says. The trend might stem in part from changes in the scientific enterprise. For example, there are now many more researchers than in the 1940s, which has created a more competitive environment and raised the stakes to publish research and seek patents. That, in turn, has changed the incentives for how researchers go about their work. Large research teams, for example, have become more common, and Wang and his colleagues have found3 that big teams are more likely to produce incremental than disruptive science.
Somewhere, I read that someone like Einstein would have never gotten anywhere in today's environment. Being a patent clerk in Switzerland and performing "thought experiments" on his own was not subject to any kind of pre-publication scrutiny that goes on today.