Food For Thought
-
Seawater.
-
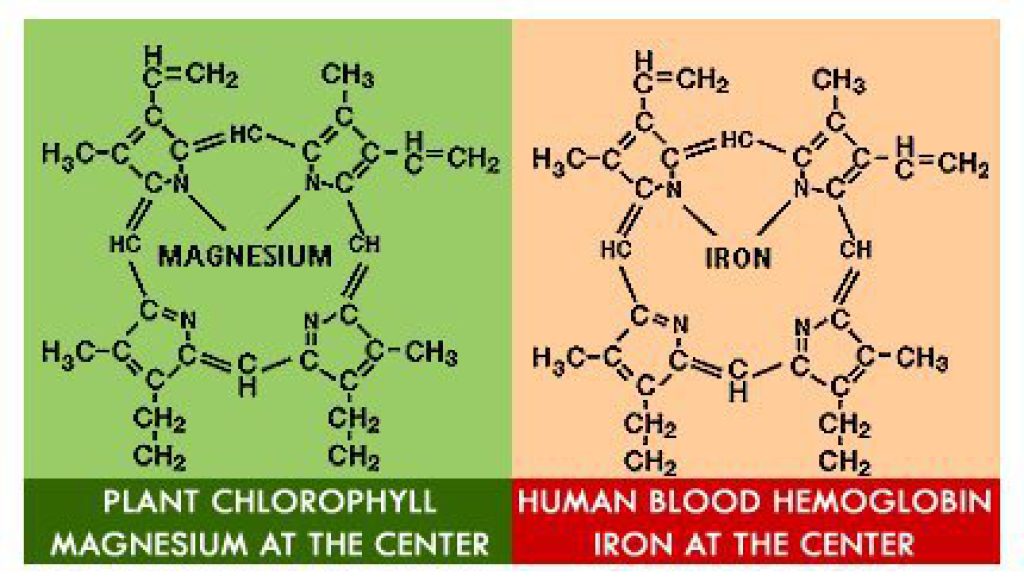
Nitpick - that's not hemoglobin, but the "heme" subunit of the hemoglobin molecule. Hemoglobin contains 4 "heme" subunits and they are surrounded by a complex protein (the globin part). I won't get into the biochemistry of oxygen transport (kinda fascinating, actually), but, the drawing is incomplete.
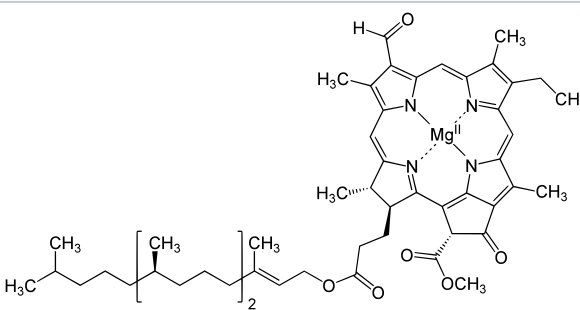
Chlorophyll is a molecule by itself, and doesn't rely on the "globin" portion that hemoglobin does for function.
In that drawing, chlorophyll is also incomplete, because it's not showing the "tail" of the molecule.
-
@Jolly said in Food For Thought:
My organic is sadly lacking. I bow in your general direction...
(descending into geekdom here, so just ignore the rest of this post)
What I always found fascinating is the way O2 is transported via the hemoglobins molecule. As I said, there are four heme groups for each molecule. The first heme group to get O2 attached to it changes the configuration, or shape, of the surrounding protein, making the deeply-buried other heme groups more exposed to oxygen, and therefore more likely to combine with the O2. The whole chemical reaction becomes much more efficient. As each O2 molecule is transported to the tissues, the process reverses, so that the loss of one O2 molecule makes chemical attraction between the other 3 O2 molecules and three other heme groups less powerful, inducing a faster deposit into the tissues.
The other things that change the affinity between O2 and Hb include temperature, CO2 content, and, perhaps most importantly, the pH of the surrounding tissue. When the pH falls, the Hb molecule is (iirc) more likely to discharge the O2 into the tissues.
If you look at the oxyhemoglobin dissociation curve, you'll see that there are, basically, four slopes to the graph, indicating the relative affinity between each successive O2 molecule and Hb.

Note that, with a "shift to the right," as in relative acidemia, hyperthermia, etc, there is less affinity between Hb and O2, and O2 is released from the Hb molecule (which is exactly what you want to happen, so that O2 is delivered to the tissues).
Fetal hemoglobin has a much higher affinity for O2 than adult hemoglobin, which also makes sense. Whatever O2 mom is carrying will be preferentially moved to the fetus.
I love this stuff.
-
@Jolly said in Food For Thought:
My organic is sadly lacking. I bow in your general direction...
(descending into geekdom here, so just ignore the rest of this post)
What I always found fascinating is the way O2 is transported via the hemoglobins molecule. As I said, there are four heme groups for each molecule. The first heme group to get O2 attached to it changes the configuration, or shape, of the surrounding protein, making the deeply-buried other heme groups more exposed to oxygen, and therefore more likely to combine with the O2. The whole chemical reaction becomes much more efficient. As each O2 molecule is transported to the tissues, the process reverses, so that the loss of one O2 molecule makes chemical attraction between the other 3 O2 molecules and three other heme groups less powerful, inducing a faster deposit into the tissues.
The other things that change the affinity between O2 and Hb include temperature, CO2 content, and, perhaps most importantly, the pH of the surrounding tissue. When the pH falls, the Hb molecule is (iirc) more likely to discharge the O2 into the tissues.
If you look at the oxyhemoglobin dissociation curve, you'll see that there are, basically, four slopes to the graph, indicating the relative affinity between each successive O2 molecule and Hb.

Note that, with a "shift to the right," as in relative acidemia, hyperthermia, etc, there is less affinity between Hb and O2, and O2 is released from the Hb molecule (which is exactly what you want to happen, so that O2 is delivered to the tissues).
Fetal hemoglobin has a much higher affinity for O2 than adult hemoglobin, which also makes sense. Whatever O2 mom is carrying will be preferentially moved to the fetus.
I love this stuff.
@George-K said in Food For Thought:
perhaps most importantly, the pH of the surrounding tissue. When the pH falls, the Hb molecule is (iirc) more likely to discharge the O2 into the tissues.
Does the pH in the body decrease as the oxygen decreases, so that a greater chance of oxygen transfer occurs?
Second question - Do all areas of the human body run at the same pH?
Interesting stuff!! Thanks for sharing!!
-
@George-K said in Food For Thought:
perhaps most importantly, the pH of the surrounding tissue. When the pH falls, the Hb molecule is (iirc) more likely to discharge the O2 into the tissues.
Does the pH in the body decrease as the oxygen decreases, so that a greater chance of oxygen transfer occurs?
Second question - Do all areas of the human body run at the same pH?
Interesting stuff!! Thanks for sharing!!
@taiwan_girl said in Food For Thought:
Does the pH in the body decrease as the oxygen decreases, so that a greater chance of oxygen transfer occurs?
The pH (and other factors) of metabolizing tissues is lower (though not by much) because CO2 is being produced - carbonic acid. So it's a byproduct of metabolism, not because O2 is lower. The fact that O2 is lower in a given tissue helps the gradient, facilitating transfer from blood to tissue, but the other factors, pH, temperature, etc cooperate to make O2 "dumping" easier from Hb.
Do all areas of the human body run at the same pH?
No, however, we measure pH in the blood, which is the only easy place to measure. As to specific pH in specific tissues, I have no idea, but a SWAG would be that highly active metabolic organs (like the brain (for most of us)) have a slightly lower (though not clinically significant) pH. But again, that's just a guess. Normal pH in the blood is 7.35 to 7.45.
Brain: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3982091/
Cerebral tissue pH is normally regulated at ∼7.0 to 7.2 pH units by the cooperative action of a multitude of sensors and regulators within cellular compartments.1 Deviation from pH homeostasis is an indicator of disease and occurs in stroke when metabolic demand for oxygen exceeds supply. Lactic acidosis leads to irreversible cellular damage in ischemic tissue (IT).2 Therefore, pH is a potentially important and sensitive fundamental measure of metabolic state and disease progression. However, few methods measure absolute pH in vivo.3
Muscle: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2279011/
The present study demonstrated that interstitial pH is continuously decreasing during muscle activity. The exercise-induced reduction in interstitial pH was correlated with power output and at high exercise intensities it was larger than the pH reduction of femoral venous blood.
-
@taiwan_girl said in Food For Thought:
Does the pH in the body decrease as the oxygen decreases, so that a greater chance of oxygen transfer occurs?
The pH (and other factors) of metabolizing tissues is lower (though not by much) because CO2 is being produced - carbonic acid. So it's a byproduct of metabolism, not because O2 is lower. The fact that O2 is lower in a given tissue helps the gradient, facilitating transfer from blood to tissue, but the other factors, pH, temperature, etc cooperate to make O2 "dumping" easier from Hb.
Do all areas of the human body run at the same pH?
No, however, we measure pH in the blood, which is the only easy place to measure. As to specific pH in specific tissues, I have no idea, but a SWAG would be that highly active metabolic organs (like the brain (for most of us)) have a slightly lower (though not clinically significant) pH. But again, that's just a guess. Normal pH in the blood is 7.35 to 7.45.
Brain: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3982091/
Cerebral tissue pH is normally regulated at ∼7.0 to 7.2 pH units by the cooperative action of a multitude of sensors and regulators within cellular compartments.1 Deviation from pH homeostasis is an indicator of disease and occurs in stroke when metabolic demand for oxygen exceeds supply. Lactic acidosis leads to irreversible cellular damage in ischemic tissue (IT).2 Therefore, pH is a potentially important and sensitive fundamental measure of metabolic state and disease progression. However, few methods measure absolute pH in vivo.3
Muscle: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2279011/
The present study demonstrated that interstitial pH is continuously decreasing during muscle activity. The exercise-induced reduction in interstitial pH was correlated with power output and at high exercise intensities it was larger than the pH reduction of femoral venous blood.
@George-K Thanks!